Ask Professor Zinc
June 2024
Q. “Are rusting guardrails a good idea? Can weathering steel safely weather the elements?”

A: Research says, “No.” While galvanized steel is widely used and known for its durability, longevity, and low maintenance, some specifiers choose non-galvanized steel, called “weathering steel” or “rusting rail,” for aesthetic reasons. So, what are the main differences between weathering steel and galvanized steel? And which is the better choice for your projects? Keep reading to find out!
Unlike galvanized steel, which is protected by an anti-corrosive layer of zinc that creates a coating harder than the steel itself, weathering steel is left bare. It has been chosen for roadside guardrails and structural elements, such as bridge girders, because the brown patina, or coating of rust, that forms on the surface is considered by some people to be more visually appealing in certain outdoor environments. Purveyors of weathering steel even say the rust “protects” the steel.
The fact is, however, that this “patina” of rust is actually corrosion, weakening the steel rather than strengthening it. Exposure to chemical corrosives such as road salts and pollution, as well as rain, snow, dirt, mud, sun, shade, humidity, and temperature can all increase the rate of corrosion and greatly shorten the life span of “rust rail” installations.
A 2009 survey conducted by the New Hampshire Department of Transportation (NHDOT) studied the conditions of weathering steel guardrails that had been in service for 10 to 20 years. It discovered a shocking overall failure rate of 25% at the midspan and a failure rate between 50%-71% at connection points. The report also noted that galvanized steel guardrails exposed to the same environments, with an equal number of years in service, showed no decrease in strength or function. The galvanized rail also became less shiny over time, allowing it to better blend into its environment.
Mike Hazlett, a Senior Engineer with NHDOT, responded to these findings by stating: “Without an intensive maintenance/replacement commitment, this (weathering steel) rail all too quickly moves from being a physical barrier to a visual barrier as far as effect on errant vehicles.” As a result, New Hampshire is one of several US states that has chosen not to allow the use of weathering steel guardrails along its roadways.
Although there are states that permit the use of weathering steel guardrails, many, including California, do not consider it to be a sustainable or safe choice in areas that receive eight or more inches of precipitation per year. (Download the California research summary here.)
According to the most recent available American Community Survey (a yearly review conducted by the U.S. Census Bureau) average annual precipitation in the United States is 30.28 inches, with the highest recording state receiving 59.15 inches of precipitation and the lowest recording state receiving 9.46 inches of precipitation. Based on this data, the use of weathering steel guard rails or bridge components should only be considered for the driest environments in the most arid states.
To better protect your projects, your people, and your budget in almost any environment, the proven choice is galvanized steel.
Click here to read what the Federal Highway Administration has to say on the subject.
We hope this answer raises awareness of an important safety issue. We’ll be writing more about this issue in the future.
What’s Your Question? Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
Ask Professor Zinc
February 2024
Q. “I was surprised to see this galvanized steel handrail near the beach in northern Florida. It looks great, but I’d heard zinc coatings weren’t suitable for marine environments. Is that not true?”


A. Who told you that? A paint salesman? No, it’s not true. When galvanized steel products are exposed to salt in normal use, such as in marine or road salt environments, the zinc forms a protective patina of mainly zinc carbonate, plus zinc hydroxide and zinc oxide. This creates a barrier that resists attack by salt and other elements and ensures long-term corrosion protection.
Take a look at the Time to First Maintenance chart below. Time to first maintenance (TFM) is defined as 5% rusting of the base steel surface, which means 95% of the surface has some zinc coating remaining, and an initial maintenance is recommended to extend the life of the structure.
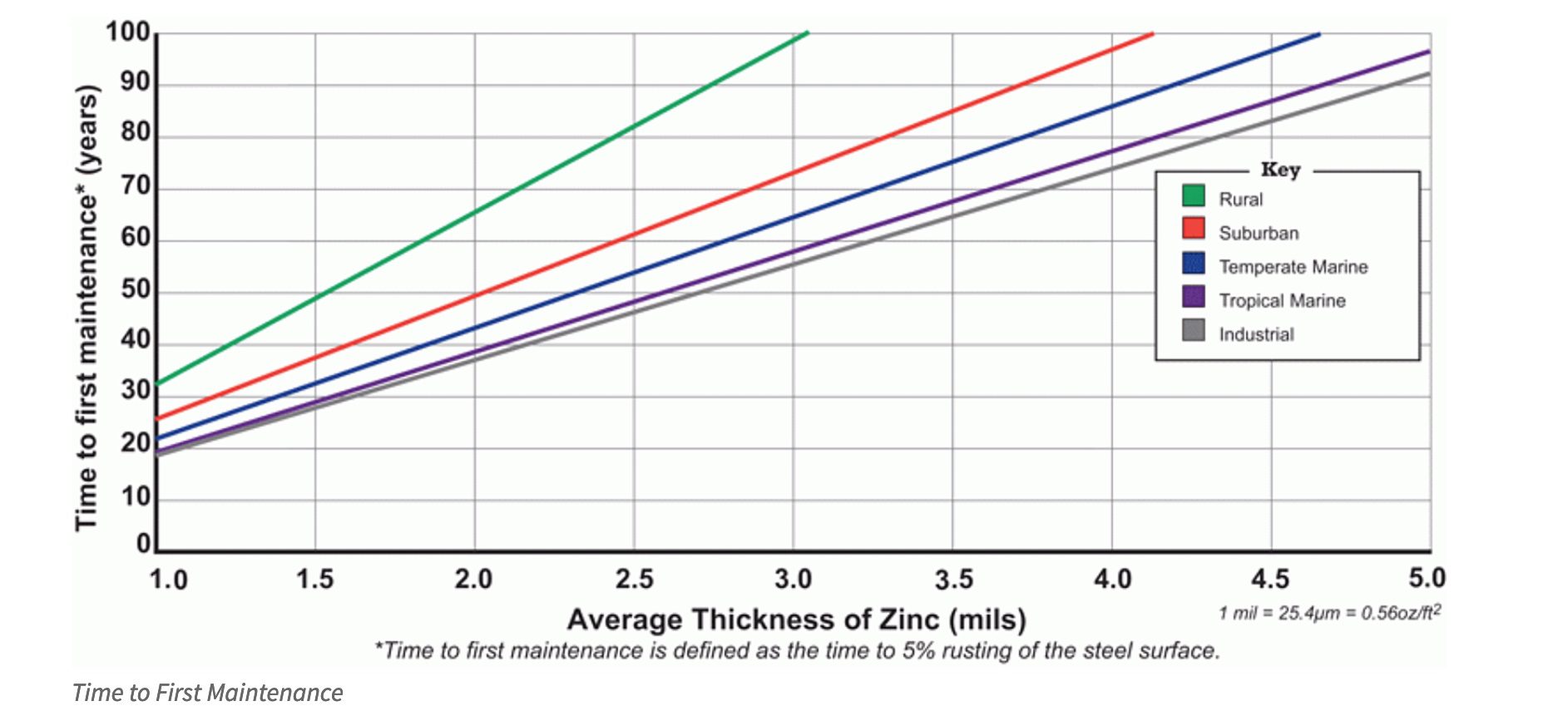
According to ASTM A123, the governing specification for hot-dip galvanizing, steel ¼-inch thick or greater must have at least 3.9 mils of zinc on the surface, but more often than not, there will be greater than the minimum requirement. Time to first maintenance for galvanized steel is linear and directly related to the zinc coating thickness. Therefore, using the TFM chart, hot-dip galvanized steel >1/4-inch thick that meets ASTM-A123 will provide 70 years or more of service life before first maintenance, even in the most corrosive atmospheres, including marine and industrial environments.
The data from the TFM chart is based on hot-dip galvanized steel’s actual performance in the field, using real-world data that’s been collected since the 1920’s. Below is a list of locations from which the data is taken.

Since substantial improvement in corrosion rates have been recorded in recent decades due reductions in pollution, the estimates on the TFM chart are conservative.
Do not be confused by manufacturers of other corrosion protection systems who claim their products perform as well as hot-dip galvanizing in testing, namely accelerated salt spray tests. Salt spray tests do not allow zinc to go through the normal wet and dry cycles, which are crucial to the development of the zinc patina (a key component to galvanizing’s longevity). Thus, they do not accurately represent the durability of hot-dip galvanized coatings, or any zinc coatings, for that matter.
Thanks for the great question! We hope you found the answer helpful.
Ask Professor Zinc
August 2023
Q. “We had rust-colored stains on some of our galvanized fabrications with overlapping surfaces. The zinc coating still meets spec in the stained areas, but it looks bad. What caused this?”

A. This photo shows the need to carefully follow ASTM A385/385M Standard Practice for Providing High-Quality Zinc Coatings (Hot-Dip), also known as the Design Spec, when fabricating steel for galvanizing. Doing so will improve the finished appearance of the job and make for a safer work environment in the galvanizing plant.
When the plate, with holes drilled through it, was attached to the channel, the outside edges of the plate were seal-welded, but the inside edges the holes were not. During the cleaning process (degreasing, rinse, pickling, flux) fluids seeped through unsealed inner edges of the holes and were trapped there between the plate and the channel.
In the galvanizing kettle, immersion in the 850˚F molten zinc vaporized the trapped fluids, pushing them back out through the holes, leaving the iron oxide stains behind.
Where surfaces overlap, and the overlapped area is less than 16 sq.in., there are a couple of welding options that meet ASTM A385 and will prevent this problem. One is to provide a gap of at least 3/32-inch (2.5 mm) between the overlapped pieces using an intermittent fillet weld on all sides so that no pocket is formed. This will ensure that all fluids drain out before galvanizing and that the area will be wetted by the molten zinc in the kettle. However, this type of welding may not be suitable for load-bearing members.
In situations where a 3/32-inch gap between overlapping pieces is not suitable, it is important that all edges be completely sealed by welding. If there is any opening, less viscous cleaning solutions will enter the gap but zinc will not. Trapped solutions may cause iron oxide to weep out of any gaps in the joint. Incomplete welds caused the problem in the photos below.


The outcome could also be much worse. Penetration of moisture into the sealed cavity could cause significant safety hazards as the sealed air will greatly expand when the part reaches the galvanizing temperature. This gas expansion can cause the molten zinc to splash out of the bath and endanger galvanizing workers.
Also note that vent holes may be required by ASTM A385 when the overlapped area is greater than 16 sq.in, depending on the thickness of the steel. See the chart below.
| Vent Holes for Overlapped Areas for Steels 1⁄2 in. (12.75 mm ) or Less in Thickness | Vent Holes for Overlapped Areas for Steels Greater than 1⁄2 in. (12.75 mm) in Thickness |
|||
|---|---|---|---|---|
| Overlapped Area in2 (cm2) | Vent Holes | Unwelded Area | Vent Holes | Unwelded Area |
| under 16 (103) | None | None | None | None |
| 16 (103) to under 64 (413) | One 3/8 in (1 cm) | 1 in (2.5 cm) | None | None |
| 64 (413) to under 400 (2580) | One 1⁄2 in (1.25 cm) | 2 in (5.1 cm) | One 1⁄2 in (1.25 cm) | 2 in (5.1 cm) |
| 400 (2580) and greater, each 400 (2580) | One 3⁄4 in (1.91 cm) | 4 in (10.2 cm) | One 3⁄4 in (1.91 cm) | 4 in (10.2 cm) |
Hot-dip galvanizing is a proven corrosion protection system that extends the maintenance-free life of steel in nearly every environment. Following the best design practices as shown in ASTM A385 for items to be hot-dip galvanized will help ensure the highest coating quality and prevent dangerous accidents.
Please contact Galvan in advance for assistance or advice on fabrications for your next product or project.
Thank you for your question. I hope you found the answer helpful. The basis information for this answer is available in a brochure from the AGA website. Click here for a free download.
Thanks again!
——-
What’s Your Question? Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
Ask Professor Zinc
April 2023
Q. “There appears to be white rust on some of the galvanized steel stored at our job site. Is this a problem?”

A. The short answer is “Probably not, unless it is severe.” Hot dip galvanized steel is carefully scrutinized during the inspection process for any issue that might indicate a problem with coating thickness. If your steel was galvanized at Galvan Industries, it passed inspection before it shipped. We guarantee our work to meet ASTM A123 specifications.
The surface condition known as wet storage stain, often referred to as white rust, is not uncommon after galvanizing. Wet storage stain is a visible formation of zinc oxide and zinc hydroxide on the surface of the galvanized steel.
You may find wet storage stain on nested or stacked products where moisture gets trapped between the articles restricting adequate airflow, or when galvanized steel is exposed to rain, dew, or high humidity conditions. The hot-dip galvanizing process does not contribute to the formation of wet storage stain. The stain occurs after the coating reacts with the environment, not during the coating process.
Wet storage stain is often superficial, even when some chalky, white product is visible on the surface. Light and medium wet storage stain does not require cleaning and does not effect the service-life of the zinc coating. Light and medium wet storage stain can be left to weather naturally as long as it will have adequate airflow. In time, the stain will convert to zinc carbonate as it reacts with carbon dioxide, completing the development of the zinc patina.
However, if airflow will be restricted at the site of installation, or the surface is likely to have standing water on it, the wet storage stain should be removed first by brushing with a stiff-bristled, nylon (not wire) brush. Then the part can be installed and exposed to the service environment.
If heavy white deposits or red stains form due to prolonged exposure to poor conditions, the damaged areas should be repaired according to ASTM A780, Standard Practice for Repair of Damaged and Uncoated Areas of Hot-Dip Galvanized Coatings. In extreme cases, wet storage stain becomes black indicating a significant amount of the zinc coating has been consumed, and the steel must be stripped and re-galvanized to meet ASTM specification requirements.
Removing Wet Storage Stain
To remove wet storage stain, brush with a stiff-bristled, nylon brush and a cleaning solution. The American Galvanizers Association (AGA) conducted a study to determine which cleaning products affect the coating finish the least. There were five products identified that do not damage the coating appearance:
- CLR®,
- lime juice,
- Naval Jelly® Rust Dissolver,
- Picklex® 10G,
- white vinegar.
For more information on the study and results, contact the AGA for the Galvanizing Note “Cleaning Wet Storage Stain from Galvanized Surfaces.”
After removing the stain, the surface should be rinsed with tap water and dried. Finally, it is best to measure the zinc coating thickness after cleaning to ensure an adequate coating remains on the base steel.
Thank you for your question. I hope you found the answer helpful. The basis information for this answer is available in a brochure from the AGA website. Click here for a free download.
Thanks again!
——-
What’s Your Question? Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
Ask Professor Zinc
August 2022
Q. “Is there any way to increase zinc coating thickness when galvanizing low-silicon steel?”

A. One step that can help increase coating thickness is to blast clean the steel prior to hot-dip galvanizing.
During galvanizing, the zinc coating formed on low-silicon steel (less than 0.04% silicon content) is limited by the interdiffusion of iron and zinc. Blast cleaning creates an easier diffusion path for the iron and zinc to mix on the surface of the steel. This allows for a thicker than normal zinc coating. We guarantee that your finished steel will meet ASTM A123 requirements.
Blast cleaning is one of several value-added services provided by Galvan. Our computer-controlled blast cleaning process uses steel shot that produces a smooth, clean surface ready for galvanizing or painting. Our blast cleaning cabinet accommodates materials up to 36″ in diameter in any length.
For work pieces too large for the shot blast cabinet, we also offer hand sand blasting with abrasive grit.
Thanks for the great question. And remember, for thicker zinc coating when galvanizing low-silicon steel, ask Galvan to blast it!
——-
What’s Your Question? Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
Ask Professor Zinc
April 2022
Q: “What can I tell a customer who is worried about graffiti removal from galvanized steel?”

Answer:
Graffiti is not as problematic for hot-dip galvanized (HDG) steel as it is for traditional paint systems used for corrosion protection. Graffiti can be easily cleaned from galvanized steel.
In fact, graffiti can sometimes be removed from hot-dip galvanized steel with simple power washing, because the graffiti paint won’t adhere well to the zinc coating without surface preparation.
And, because the zinc is metallurgically bonded to the steel, the HDG coating withstands scrubbing and chemical cleaners to eliminate graffiti without fear of scratching or degrading corrosion protection.
Scrubbing with an aggressive solvent (acetone, MEK, commercial paint removers/thinners) or alkaline cleaners will remove graffiti from hot-dip galvanized steel. Abrasive pads should not be used for scrubbing, however, as this may result in removal of some zinc at the surface of the coating and could affect the long-term corrosion protection.
Although short exposure to chemical cleaners will not affect corrosion prevention, it is important to rinse the area thoroughly with fresh water immediately after cleaning to remove any chemicals remaining on the surface.
Thanks for your question. We hope you found the answer useful.
Ask Professor Zinc
February 2022
Q: “What is the best way to handle field repair of galvanized coating damaged at the construction site?”



Answer:
There’s an ASTM spec for that, and lots of available information.
ASTM A780 Practice for Repair of Damaged and Uncoated Areas of Hot-Dip Galvanized Coatings details how to repair a damaged hot-dip galvanized coating. The specification explains how to use the various repair methods as well as the required coating thickness for the repaired area. Touch-up materials are required to meet a coating thickness of at least 2.0 mils (50.8 µm) for one application, and the final coating thickness of the repair area is dictated by the material used to do the repair.
ASTM A780 contains three acceptable methods of touch-up and repair of hot-dip galvanized steel: zinc-based solders, zinc-rich paints, and zinc spray (metallizing). Detailed information and videos about each of these methods are available on the American Galvanizers Association web site.
Although the hot-dip galvanized coating is very resistant to damage, small voids or defects in the coating can occur during the galvanizing process or due to improper handling of the steel after galvanizing. Touch-up and repair of galvanized steel is simple whether newly galvanized or in service for years. The practice is the same, but there are more restrictions to the allowable repairs on a new product than one that has been in service.
The main restriction in the specification for repairing newly galvanized material is the size of the area which is outlined in the product galvanizing specifications. For example, ASTM A123 Zinc (Hot-Dip Galvanized) Coatings on Iron and Steel Products allows repairs on newly galvanized materials with the following restrictions:
- One inch or less in narrowest dimension
- Total Area can be no more than 1/2 of 1% of the accessible area to be coated or 36 sq.in. per short ton per piece, whichever is less
When it comes to repairing galvanized steel in the field, there is no limitation to the size that can be repaired. The zinc coating is difficult to damage, but field fabrication that requires removal of the coating should be minimized as much as possible. The cathodic protection of the coating will provide some protection to uncoated areas, but the best practice for longevity is to touch-up any bare areas per ASTM 780 specifications.
Thank you for your question! We hope you found the answer helpful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
Ask Professor Zinc
October 2021
Q: “Can you provide a warranty for the expected service life of galvanized steel?”
Answer:
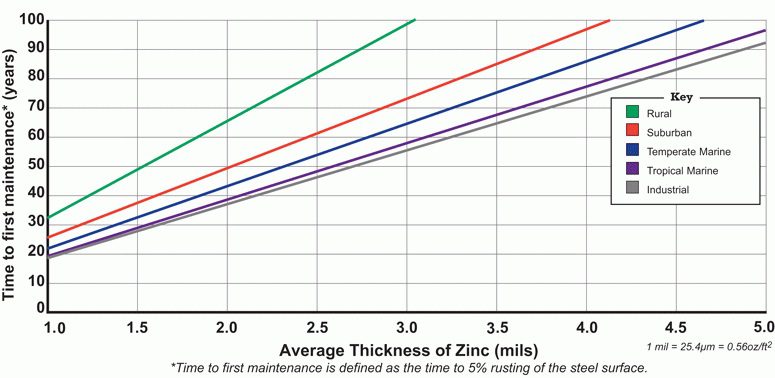
Like all other providers of hot dip galvanizing, we do not issue warranties, but we do guarantee that your product or project will be galvanized to ASTM A 123/A which requires that a minimum zinc coating thickness be applied to the steel. Because of differences in steel composition and the variables involved in the galvanizing process, it is likely that more zinc will be applied to the steel than the ASTM minimum. This means that your product/project will have excellent lifetime corrosion resistance.
To help gauge likely performance, the American Galvanizers Association (AGA) has published a Time To First Maintenance Chart, shown above. ‘Time to first maintenance’ is defined as 5% rusting of the steel surface. The chart provides estimates of the time to first maintenance based on coating thickness and the environment in which the steel will be used. The coating thickness of hot-dip galvanized steel is commonly between 4 and 5 mils.
The Time To First Maintenance Chart was developed using a corrosion prediction model called the Zinc Coating Life Predictor (ZCLP). developed by Dr. Gregory Zhang of Teck Metals, based on galvanized steel performance data collected in various environments around the world. Your product/project performance may vary dramatically from that suggested, and since the chart is conservative, lifetimes may be significantly longer. Conversely, if there are unique environmental conditions such as proximity to concentrated sulfates, stray electrical currents and extreme temperatures; contact with incompatible metals; immersion in tidal waters; contact with soil of unknown composition, lifetimes may not be as long as indicated.
You can use the ZCLP yourself. Just click here and put in your specific parameters. Obviously, atmospheric levels of airborne salinity, precipitation, relative humidity, sulfur dioxide, and temperature influence actual corrosion rates in specific geographic locations.
Annual average temperatures, precipitation and humidity for your state or city can be found at www.currentresults.com. You may have to estimate airborne SO2 and salinity levels for your site unless you have actual test results. The following information might help in your estimating. According to the European Community’s LIFECON project (2003), concentrations of airborne sulfur dioxide from 60 mg/m2/day and up are considered Industrial, with 10 to 80 mg/m2/day being Light Industrial, and below 10 mg/m2/day being benign. Similarly, 60 or more mg/m2/day of airborne salinity is considered a Marine environment, with 15 to 60 mg/m2/day being Light Marine and below 15 mg/m2/day being benign. For coating thickness, since hot dip galvanizing is usually between 4 and 5 mils thick, I would go with 4.5. (Be sure to select ‘mils’ from the dropdown.)
Plug in the parameters that best describe your project and see what the ZCLP has to say. Please note that some of the parameters have drop-down selections. Make sure you choose the right ones. It will affect the results.
Thank you for your question! We hope you found the answer helpful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
Ask Professor Zinc
August 2021
Q: “What should be the weight of zinc for maximum protection for steel in a saltwater environment?”
Answer:

The weight of the zinc on any article after galvanizing depends on the thickness of the article being galvanized. Normally, galvanizing will add 4% to 8% to the nominal weight of a steel article. Heavier items normally pick up a lower percentage of weight (4%-5%) than thinner material. Lighter, thinner materials pick up a proportionally heavier percentage, and usually show a 7% to 8% increase.
Here’s why. Hot dip galvanizing forms a metallurgical bond between steel article and the zinc in the galvanizing kettle. The coating “grows” from the surface of the steel, and the rate of growth slows as the steel reaches the temperature of the galvanizing bath. Thin materials heat rapidly. Thicker materials take longer to heat and develop thicker coatings. The specifications for galvanizing are covered by ASTM Iron A123 Standard Specification for Zinc (Hot-Dip Galvanized) Coatings on and Steel Products.
The following tables reflect the minimum average coating thicknesses required by ASTM A123.
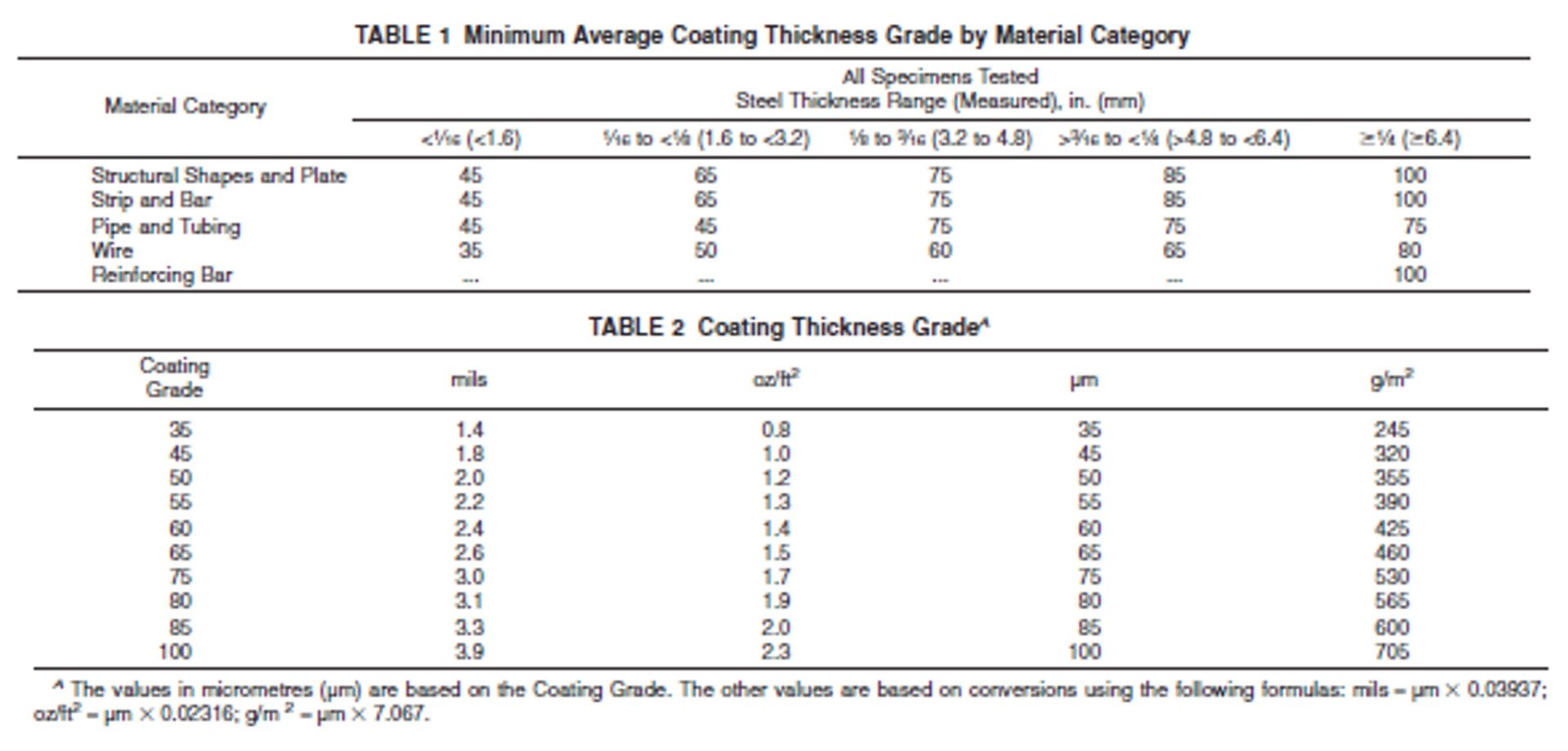
Structural steel at least ¼” thick should develop a minimum of 3.9 mils on any given surface. That’s the ASTM A-123 Minimum Average Coating required for this class of material. Your galvanizer must meet this standard, but due to steel thickness and the reactivity of certain steels, it is not unusual to exceed the minimum and provide a slightly heavier (thicker) zinc coating.
Since zinc galvanizing service life is proportional to coating thickness, heavier coatings last longer than thinner coatings in any environment, including proximity to salt water.
I hope this answer helps. Thank you for your question! We’re always happy to provide advice to help you get the best galvanizing possible.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
Ask Professor Zinc
April 2021
Q: “We are contacting Galvan in hopes that you could suggest a fix for the poles that were galvanized elsewhere and then powder coated (over the galvanized coating) in the attached pictures. The powder coating appears to be flaking off along with the galvanized coating. What is causing this, and can you re-galvanize these parts for us?”
Answer:


Before I answer your final question, we need to clarify the problem. Based on your photos, the powder coating is not the issue. The photos show delamination of the top layer of the galvanized zinc coating due to a phenomenon known as the Kirkendall effect.
Named after Ernest Kirkendall, an assistant professor of chemical engineering at Wayne State University in the 1940s, the Kirkendall effect refers to the motion of the interface between two metals in an alloy due to the difference in diffusion rates of the metal atoms. The Kirkendall effect comes into play negatively in galvanizing when steel is allowed to cool too slowly after being removed from the zinc bath. It is the primary cause of peeling or delamination on heavy steel parts after galvanizing.
During the hot-dip galvanizing process, steel is heated to approximately 830 F (443 C) in the molten zinc bath of the galvanizing kettle. The zinc in the kettle is maintained at that temperature.
While immersed in the kettle, the iron in the steel reacts with the zinc to form a series of protective zinc-iron intermetallic alloy layers. Once the item being dipped reaches the same temperature as the bath, it is withdrawn. The metallurgical reaction continues as long as the steel remains near bath temperature.
Heavier steel will retain heat longer, allowing the galvanizing reaction to continue longer. This can cause voids to form between the top layer of solidified zinc and the hot steel core. These are referred to as Kirkendall voids. If a large number of voids are formed, the top layer of zinc can separate from the rest of the coating and crack or peel off. That is what we are seeing in your photos.

Steel removed from the zinc bath should be immediately dipped in the quench tank to stop the galvanizing reaction. If this is not possible, it should be thoroughly sprayed with cold water to reduce its temperature. It appears these steps were not taken. The result is the delamination of the surface zinc, with a rough textured gray finish underneath.
The surface appearance doesn’t necessarily mean that the material is unusable. In most cases, if the remaining coating still meets the minimum ASTM specification requirements for thickness, then it would be considered acceptable. In your case, however, since the galvanizing was intended as a base coat for a galvanized/powder-coated duplex system, you would be right to reject it.
Now to your question: can it be fixed? Yes, but it will be expensive and time-consuming. First, it will need to be sent back to the powder coating company to have the coating baked off. Afterward, when we receive it, we will remove all remaining zinc coating chemically and properly re-galvanize the steel. Then it can be returned to the coater to be powder coated again. We’ll be happy to help you solve this problem. The big take-away from your experience, though is this: “Next time, call Galvan first!”
Thanks for the question! Let us know if you need help with transportation to our plant.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
Ask Professor Zinc
February 2021
Q: “We are fabricating a piece with 6” x 2” x 39” long capped rectangular tube welded to a steel beam. What is the correct size and placement of vent holes on the tube section?”
Answer:
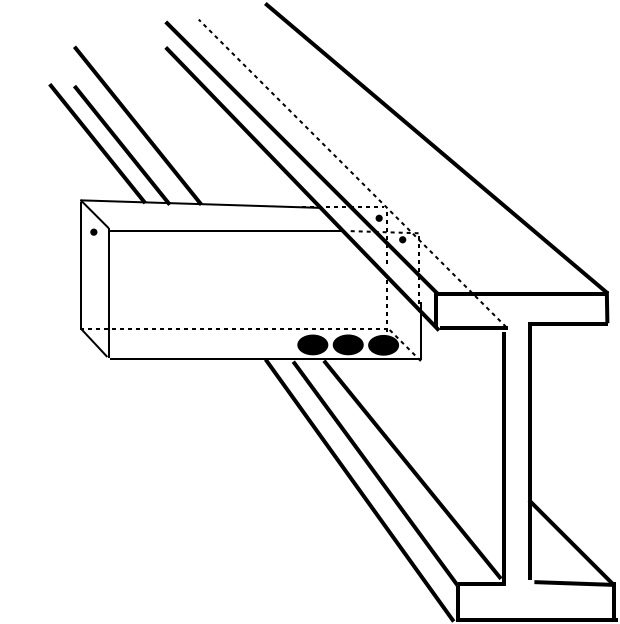
The size of vent openings for a rectangular tube or box section depends the size of the tube. The smaller the area of the tube, the larger percentage of that area must be allowed for venting.
According to 385/A385M − Standard Practice for Providing High-Quality Zinc Coatings (Hot-Dip), the following formulas apply to venting box sections.
Box Sections—H + W = 24 in. [610 mm] or larger-Area of hole plus clips shall be at least equal 25 % of the area of the box (H x W).
Box Sections—H + W less than 24 in. to and including 16 in. [384 mm]-use 30 %.
Box Sections—H + W less than 16 in. to and including 8 in. [192 mm]-use 40 %.
Box Sections—H + W under 8 in. leave completely open; no end plates or internal gussets.
In your case, the area is 12 sq. in., which means the vent openings must equal a minimum of 4.8 sq. in. (40%).
The best arrangement of the vent holes would be three equal size holes with a total area of 4.8 inches on the bottom side of the tube. These holes will allow the molten zinc to fill and drain from the tube. Two additional ½-inch diameter holes are needed at the top corners of the tube where the flanged end connects with the beam, to prevent air pocketing in that area during the hot dip galvanizing process. Another ½-inch diameter hole is needed at the top center of the tube cap, also to allow air to escape during dipping.
For quality galvanizing, inside and out, always follow ASTM 385/A385M for venting and draining your fabrication.
Thank you for your question! We’re always happy to provide advice to help you get the best galvanizing possible.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
Ask Professor Zinc
August 2020
Q: “Is there any special surface prep required for steel before it’s sent to a galvanizing plant?”
Answer:

There is no special surface preparation required for hot dip galvanizing under ASTM specifications, but some things that cause problems during galvanizing should be removed or cleaned off before your steel is sent to the plant. This includes:
- Weld slag and other welding flux residues
- Weld splatter and anti-splatter
- Burrs (could include excessively rough edges from flame cutting)
- Very heavy or extremely adherent mill scale
- Mill coatings such as varnishes or lacquers present on some types of pipe
- Epoxies, types of vinyl, and asphalt
- Sand and other impurities on castings
- Oil-based paints and markers
- Crayon markers
- Very heavy or thick deposits of wax or grease
In the absence of these problems, no surface preparation is required on the customer’s part. Galvan Industries includes the normal preparation of steel or iron as part of the galvanizing process.
After we inspect the steel to ensure it has adequate vent and drain holes, we dip it into a series of cleaning chemicals.
The first chemical is a degreasing bath that removes organic contaminants such as dirt, grease, and oil from the metal. The next chemical used is pickling acid, which removes mill scale and rust (oxides) from the steel.
The last step before galvanizing is dipping the steel or iron into a flux bath, which prevents oxidation of the metal prior to entering the galvanizing bath and also aids the reaction developing the hot-dip galvanized coating.
Thanks for the great question. We hope you find this answer helpful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
Ask Professor Zinc
May 2019
Q: “Is any special preparation required for painting over galvanized steel?”
Answer:

Painting over hot-dip galvanized steel, known as a duplex system, has been used for decades as a means to enhance corrosion protection while allowing paint to be used for color coding or aesthetic reasons. Duplex systems are becoming increasingly common and popular because of this combination of benefits.
Successfully painting over hot-dip galvanized steel is relatively simple, but like painting on any other surface, proper surface preparation is key to creating an effective bond between the paint and the galvanized surface.
Follow ASTM D 6386-99 Standard Practice for Preparation of Zinc (Hot-Dip Galvanized) Coated Iron and Steel Product and Hardware Surfaces for Painting.
The basic steps before painting are as follows:
1. Talk to the galvanizer the make sure they know your plans and can avoid any treatments that would impede paint adhesion.
2. Determine the condition of the surface: newly galvanized, partially weathered or fully weathered.
3. Clean the surface. Remove bump, runs and drips and remove any organic materials. Then rinse and dry.
4. Profile the surface. This final step may include sweep blasting, a wash primer, acrylic pretreatment and/or surface grinding.
Proper surface preparation is critical, but the final result is worth it when the two coatings work together in synergy.
The exterior layer of paint or powder coating will slow down the rate at which the zinc is consumed, greatly extending the life of the galvanized steel. In return, once the exterior layer has been weathered down or damaged, the zinc beneath is still available to provide cathodic and barrier protection. As a result, the substrate steel is afforded corrosion protection for 1.5 to 2.3 times the sum of the expected life of each system alone.
For example, if a galvanized coating alone on black steel would provide 50 years of maintenance-free protection and a paint coating would not require any maintenance for 10 years, the combination duplex system would provide maintenance-free protection for 90 to 138 years in the same environment. A periodic maintenance schedule can extend this synergistic lifetime even further.
Thanks for the great question! We hope you find the answer useful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
February 2019
Q: “Can muriatic acid be used to clean hard water mineral deposits off galvanized steel parts in an irrigation system?”
Answer:

Muriatic acid is another name for hydrochloric acid. Diluted hydrochloric acid is often used as a descaling agent to remove limescale from metal surfaces in contact with hot water, such as in boilers, water heaters, and kettles. However, it disolves zinc. Zinc reacts quickly with the acid to form bubbles of hydrogen and zinc chloride, a salt. Because of this reaction, muriatic acid is actually used in a 20% solution to remove zinc coatings from steel that has to be welded after galvanizing. So the answer is ‘no’ for using muriatic acid for cleaning galvanized parts.
CLR Calcium Lime Rust Remover would be a better choice for your task. It will remove hard water calcium, lime and iron deposits without dissolving or damaging the protective zinc coating on your irrigation system components.
If for any reason you need to touch up or repair a galvanized surface, one simple method is to use a zinc-rich paint such as Rustoleum Cold Galvanizing Compound. It can be applied to a clean, dry steel surface by either a brush or spray. Zinc-rich paints must contain either between 65% to 69% metallic zinc by weight or greater than 92% metallic zinc by weight in dry film. The coating thickness for the paint must be 50% more than the surrounding coating thickness, but not greater than 4.0 mils. Measurements should be taken with either a magnetic, electromagnetic, or eddy current gauge to ensure compliance.
Thank you for the great question. We hope you find this answer helpful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
October 2018
Q: “Is it possible to take steel which was recently galvanized, and re-galvanize without stripping the old coating off? The problem is bare spots from welding changes.”
Answer:

The short answer is “No”. Based on your description of the problem, welding through galvanizing has removed the coating at the weld area and in the surrounding heat affected zone of the weld. Zinc melts at 786˚C and during welding produces a voluminous white zinc oxide at about 1000˚F. Welding temperatures are in the range of 3-4000˚F or higher, depending upon the method.
ASTM allows for repair, but limits the allowable area. The total area subject to renovation on each article shall be no more than 1⁄2 of 1% of the accessible surface area to be coated on that article, or 36 in.2 per short ton [256 cm2 per metric ton] of piece weight, whichever is less.
The specification further states, “Materials that have been rejected for reasons other than embrittlement are not prohibited from being stripped and re-galvanized and again submitted for inspection and test at which time they shall conform to the requirements of this specification.”
The hot dip galvanized coating is formed through a diffusion reaction between iron and zinc and develops a metallurgical bond between the two metals. In order for the iron-zinc alloy to develop the steel substrate must be completely clean. Residual weld fluxes, oils, paint or any other contaminant will result in a bare area that will not galvanize. Attempting to re-galvanize without removing the existing zinc will result in a coating with bare areas and zinc that is not adherent to the pre-existing coating.
Galvanized steel develops zinc oxide on the surface of the material within 24 hours of the galvanizing process. The coating gradually weathers to form zinc hydroxide and finally zinc carbonate. The re-galvanizing process will require that any zinc on the steel surface be removed completely before being re-coated in order to meet ASTM specifications.
Thanks for the great question! We hope you find the answer helpful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
August 2018
Q: “What is the best way to mark steel parts so they can be identified after galvanizing?”
Answer:


The identification of parts throughout the galvanizing process is an important consideration. Parts are marked for tracking during processing as well as to identify the project, customer, and/or assembly for shipping. Parts that are properly marked also simplify handling and construction at the job site. There are two categories of marking.
- Temporary identification – detachable embossed metal tags or printed tags especially designed for galvanizing such as the Kettle Tags® system used by Galvan (top photo), which stays on the material through all the steps in the process, including the acid dip and the molten zinc bath.
- Permanent identification – stamping, weld beads, and deep stencil marking (bottom photo)
Identification applied before galvanizing should made with a material or design that will be readable after galvanizing, but not disrupt the zinc coating’s integrity. All the methods mentioned above meet these requirements. However, the Kettle Tags system allows for more legible information and lowers the cost and time involvement for temporary ID in most applications.
Customers taking advantage of the benefits of Kettle Tags send Galvan a list of items that need to be tagged along with the information that should appear on the tag, including text, logos and/or bar codes. Galvan prints the tags and sends them to the customer to be attached to the appropriate materials before being shipped to Galvan. Once attached, the tags are not removed. They stay in place throughout the galvanizing process and through delivery to the customer or job site.
Because of the amount of information that can be conveyed and the fact that they stay where placed by the customer, Kettle Tags may be the answer to your question.
Thanks for asking. We hope you find the answer helpful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
June 2018
Q: “Is electroplating per ASTM B633 equal to hot dip galvanizing to ASTM-A385?
Answer:
No, not at all. B633 is the Standard Specification for Electrodeposited Coatings of Zinc on Iron and Steel. In general terms the zinc coating is thin, up to a maximum thickness of 1 mil (25 μm), and mechanically bonded to the surface with a hardness of about a third to a half that of most steels. The specification, ASTM B633, lists four classes of zinc-plating: Fe/Zn 5, Fe/Zn 8, Fe/Zn 12 and Fe/Zn 25 where the number indicates the coating thickness in microns (μm). Most coatings are less than a half of a mil in thickness and are intended for indoor and/or non critical applications.
ASTM A385 is not actually a coating specification but the Standard Practice for Providing High-Quality Zinc Coatings (Hot-Dip), and is commonly referred to as the “design” spec for galvanizing.
ASTM A123 Standard Specification for Zinc (Hot-Dip Galvanized) Coatings on Iron and Steel
Products and ASTM A153 Standard Specification for Zinc Coating (Hot-Dip) on Iron and Steel Hardware, are more likely the specifications you want to compare to.
Hot dip galvanized structural material will range from 45 μm to 100 μm as a minimum (1.8 to 3.9 mil) and hot dipped fasteners will range from 45 μm to 86 μm (1.7 to 3.4 mil), depending upon the type of material being coated.
Since zinc coating performance is linear and is based on coating thickness and conditions of exposure, a two mil coating will last twice as long as a one mil coating in the same environment. You can see from the relative coating thicknesses of electrogalvanizing and hot dip galvanizing that they are NOT equal. Hot dip galvanizing will have a service life that may be as much as 20 times the life of a B633 coating in the same application.
Thanks for your question and please don’t hesitate to contact us again if we can be of additional assistance.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
April 2018
Q: “What is the additional weight per square foot of surface area of galvanizing on a steel member?”
Answer:

Good question. Normally, galvanizing to ASTM standards will add 4% to 8% to the nominal weight of a steel member, depending upon the thickness of the steel being galvanized. Galvanizing is an alloy that is formed through a diffusion reaction between iron and zinc. The steel is allowed to remain in the galvanizing bath until the steel core reaches the temperature of the surrounding zinc bath, when the galvanizing reaction is complete. Thicker (heavier) steels take longer to heat, so the reaction continues for a longer period. Thinner (lighter) steels absorb heat more quickly and complete their reaction sooner and develop a lighter coating mass. It seems counter-intuitive, but heavier steels normally pick up a lower percentage of weight (4%-5%) due to the weight/surface area ratio, than thinner material. Light sections pick up a proportionally heavier percentage due to the same effect, and usually show a 7% to 8% increase in mass.
The ASTM Standards provide for MINIMUM coating weights according to the tables below:


Please remember that these are MINIMUM coating thickness standards. Galvanizing will normally meet or exceed these minimums in every case. A steel beam that has a nominal thickness of >1/4” will result in a galvanized coating of 100 micrometers, that will add a minimum of 2.3 oz/ft2 to the weight of the article.
Thanks for the question. Please don’t hesitate to write again if you need additional information.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
February 2018
Q: “Is there an appropriate/recommended methodology to obtain an acceptable non-slip surface for a galvanized steel plate deck, either during the galvanizing process or after?”
Answer:

Yes, there are actually both pre-galvanizing and post-galvanizing options.
One of the most effective and reliable methods of creating a non-slip galvanized surface is to apply a thermal metallizing spray to the steel before galvanizing. In this process, the non-slip surface is produced by applying molten or semi-molten particles of metal onto the steel plate. This creates a permanent, rough random matrix on the steel for increased traction. The bond strength of the metal particles to the steel plate is 5,000 psi or more.
The steel should be blasted to SSPC Surface Preparation Specification 10 and be clean and free of oils and grease before it is metallized. After application, the metallized steel is hot-dip galvanized in accordance with ASTM A 123, providing decades of rust free, slip-resistant service.
Another option is the application of a special non-slip paint or epoxy after galvanizing. This may be a less expensive approach initially, but could require maintenance or reapplication later, increasing life-cycle cost.
Thanks for the great question! We hope you find the answer helpful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
December 2017
Q: “Can structural steel ASTM A36 and steel pipe ASTM A53 be hot dipped galvanized to a minimum thickness of 6 mils?”
Answer:

Yes, technically it is possible, depending on the chemistry of the steel and the thickness of the material itself, but a 6-mil minimum coating thickness is well beyond the requirements of ASTM A123/123M for Structural Steel Products, which galvanizers use as a standard.
The ASTM A123/A123M specification covers individual steel pieces as well as assemblies of various classes of material. The six material categories covered include structural shapes, strip and bar, plate, pipe and tubing, wire, and reinforcing bar. Per this standard, the required minimum coating thickness for structural steel greater than 5/8-inch in thickness is 3.9 mils. For pipe greater than 5/8-inch in wall thickness, the minimum is 3.0 mils.
It is the responsibility of the galvanizer to ensure compliance with these specifications, as long as the product is in accordance with the supporting design and fabrication specifications.
It should also be noted that excessive coating thickness can cause problems. Although a thick coating is associated with a longer time to first maintenance, coatings developed beyond 8-10 mils tend to be very brittle and are susceptible to flaking both at the galvanizing plant or after delivery to the job site upon exposure to impact forces during handling, transportation, or installation by the customer.
The ASTM A123/A123M specification has stood the test of time. Sticking with a proven standard is always a good idea.
Thanks for the question! We hope you find the answer helpful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
November 2017
Q: “Why does hot dipping make welds seem to pop up?”
Answer:

The answer is most likely the difference between the chemistry of the steel and the weld metal. Galvanized coating thickness primarily depends on the silicon content of the iron or steel part. The major difference between the weld metal and the structural steel is the amount of silicon in the weld rod. Excessive silicon in the weld filler material can accelerate the growth of the hot-dip galvanized coating. Because some weld rod metal contains nearly 1% silicon, the difference between the coating thickness on the weld metal and the surrounding structural steel can be significant. Excessive silicon in the weld material to be galvanized causes an accelerated formation of the zinc-iron layers that make up the hot-dip galvanized coating, greatly increasing coating weight.
When the fabricated structure is immersed in the zinc bath long enough to achieve a coating that meets the minimum thickness of the galvanizing standards (such as ASTM A 123/ A 123M, Standard Specification for Zinc (Hot-Dip Galvanized) Coatings on Iron and Steel Products), the coating on the high-silicon weld metal can be more than two-times thicker than the surrounding coating. This thick coating on the weld detracts from the appearance of the fabricated structure and increases the possibility of the zinc coating becoming damaged in the weld area with further handling of the assembly or part.
For typical welding processes, such as shielded metal arc welding (SMAW), submerged arc welding (SAW) and flux-cored arc welding (FCAW), there are weld rod materials that will not cause excessively thick coatings.
Thanks for the question! We hope you find the answer helpful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
September 2017
Q: “Can you dip something measuring 9′ 2″ wide x 15′ long x 3′ thick? It is made of I-beams and channels. Here is a photo.”

Answer:
Absolutely. When a fabrication is too large for a single immersion, it is often possible to galvanize it by progressive dipping, immersing one end of the work at a time in the galvanizing bath. Galvan Industries’ hot dip galvanizing kettle is 42′ long x 4’6″ wide x 8’6″ deep. With “progressive” or “double dipping”, our large bath size will allow us to hot dip galvanize almost any structure for greatly reduced maintenance costs and extended life.
Click here to see our double-dipping chart.
Because hot-dip galvanizing is a total immersion process, it is always a good idea to verify kettle constraints with your galvanizer in advance when dealing with a large items like this one.
Thanks for the question! We hope you find the answer helpful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
July 2017
Q: “What is the best arrangement of vent holes in a sealed rectangular tube that is 10 inches by six inches? Length varies.”
Answer:
ASTM A385/A385M specifies that a minimum vent opening of 30% of the cross-sectional area of the tubular structure must be included on each end of the capped tube. This is so that no air or solutions are trapped inside the assembly during galvanizing. In this case, the cross-sectional area is 60 sq. in., which means the vent openings must equal 18 sq. in. (30%).
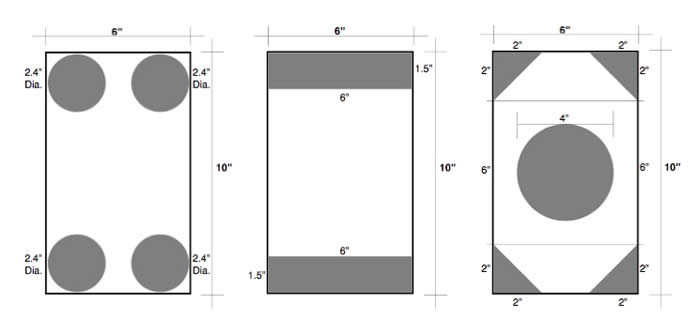
The diagrams here show three different ways to achieve the 30% vent opening. All three meet the requirements of the spec. The best arrangement is the one that works best for your situation or application.
Thanks for the great question! We hope you found the answer helpful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
May 2017
Q: “What is hot dip galvanizing CLASS B-2?”
Answer:
Class B-2 is a materials classification from ASTM A153 for hardware products such as castings, fasteners and miscellaneous threaded objects that are centrifuged, spun, or otherwise handled to remove excess zinc. Class B is refers specifically to rolled, pressed, and forged articles. B-2 identifies such articles under 3/16 inch (4.76mm) in thickness and over 15 inches (381mm) in length.
The requirements for ASTM A153/A153M are very similar to those for ASTM A123/A123M, except for the addition of threaded products and embrittlement requirements.
ASTM A153/A153M Requirements
• Finish – continuous, smooth, uniform
• Embrittlement – high tensile strength fasteners (>150ksi) and castings can be subject to embrittlement
• Appearance – free from uncoated areas, blisters, flux deposits and gross dross inclusions as well as having no heavy zinc deposits that interfere with intended use
• Adherence – the entire coating should have a strong adherence throughout the service life of hot-dip galvanized steel
• Threaded Products – areas with threads are not subject to the coating thickness requirement
• Coating Thickness/Weight – depends on the material category and steel thickness, as listed in Table 3 of the specification. For Class B-2, the requirements are as follows:
| Weight (Mass) of Zinc Coating, | Coating Thickness, mils | |||
| oz/ft2 (g/m2) of Surface, minimum | (microns), Minimum | |||
| Class of Material | Average of Specimens Tested | Any Individual Specimen | Average of Specimens Tested | Any Individual Specimen |
| B-2 | 1.5 (458) | 1.25 (381) | 2.6 (66) | 2.1 (53) |
For more information, visit the Galvanizing Standards section of the AGA web site, www.galvanizeit.org.
Thanks for the great question! We hope you found the answer helpful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
March 2017
Q: “We have a supplier of an endfitting overseas that is having trouble galvanizing the inside of a cast fitting due to a deep pocket in the fitting. I have attached a few pictures. Could you explain the difficulty of getting the galvanization to work in this area?”
Answer:


A.There are two possible explanations for the bare spots inside the castings. The first is that they were not properly cleaned before dipping. Castings must be abrasive cleaned by shot blasting prior to the galvanizing process. If there is any sand or other residue left from the casting process inside the part, it will not galvanize correctly. At Galvan, we blast clean every casting prior to galvanizing.
The second possible explanation is the formation of an air pocket inside the part. If this part is not hung at exactly the right angle when it is being dipped, an air pocket will form at the back of casting and it will not get full coverage of zinc. The air will not allow the zinc to reach the back of the part.
A fix could be an added hole through the back of the part to allow the air to escape. However, if the purpose of this part is to stop the flow of a liquid or gas, adding a hole would create another problem.
If that is the case, the answer to the problem is greater care in cleaning to eliminate sand inclusions and greater care in dipping to ensure that air inside the casting is allowed to escape.
Regardless of what caused the problem, these parts as shown should be cleaned and repaired, or rejected, stripped, and regalvanized.
Thanks for your question and good luck in resolving this issue.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!
January 2017
Q: “Does the cost of galvanizing really pay off for transportation infrastructure projects like bridges and airports?”
Answer:
A. Airports, bridges, highways and mass transit systems are major public investments. It is critical to protect these large investments with a sustainable, durable, maintenance-free corrosion protection system that will withstand the effects of constant rough usage and environmental exposure. Hot dip galvanizing the steel used in infrastructure projects from rebar and structural steel to guard rail and sign posts will lead to significant savings in maintenance and repair costs. The extended the life of projects in question will also save or delay replacement costs.
The annual direct cost of corrosion for highway bridges alone is estimated to be $6.3 to $10.15 billion, a cost that has to be paid by state and federal taxes. Building every new bridge – structural steel or reinforced concrete – with galvanized steel would keep those costs from rising further and reverse them in the future as older bridges are replaced. Optimizing the return on an infrastructure investment means building things to last as long as possible, safely and maintenance-free. Given that, galvanizing definitely pays off.
Happy New Year and thanks for the question. We hope you find the answer helpful.
What’s Your Question?
Submit your technical galvanizing question now! We’ll get back to you with the Professor’s answer ASAP. It could be featured on this page or in our next e-newsletter. Think of the honor!




